Yury Miller Lab
- Research
- Lab Members
- News
Vascular lipid accumulation and vascular inflammation are major pathogenic processes in the development of atherosclerosis. Inflammation promotes lesion growth and its development into a vulnerable plaque. But why is vascular lipid accumulation per se leading to inflammatory responses by vascular cells? The hypothesis my colleagues and I have developed proposes that lipoprotein oxidation, which inevitably occurs in lipid-rich vascular lesions, creates agonists for innate immune receptors (1).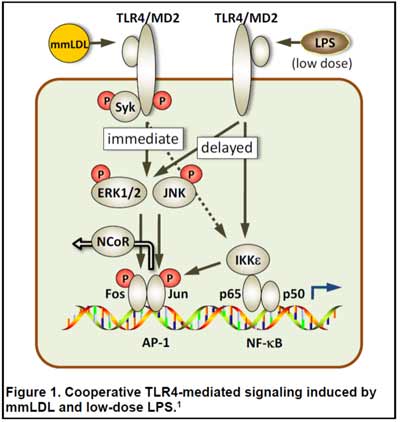
Our discovery that oxidized cholesterol esters (OxCE) induce inflammatory responses in macrophages via TLR4/MD2 (2-5) supports this hypothesis. The finding that an OxCE activates TLR4, the receptor for bacterial LPS, was further supported by the recognition of structural homology between plasma LPS-binding protein (LBP) and cholesterol ester transfer protein (CETP), and between MD2, which binds LPS, and NPC2, a lysosomal cholesterol ester binding protein. Importantly, we have shown the presence of specific OxCE moieties, which activate TLR4/MD2, in human plasma and in human coronary plaques (5,6).
Emerging evidence suggests that persistent subclinical endotoxemia is an integral component of metabolic disorders induced by Western type, high-fat diets, and has been termed "metabolic endotoxemia." We have demonstrated that injections of low doses of LPS and minimally oxidized LDL (mmLDL) cooperatively (and even synergistically) activate macrophages in a TLR4-dependent manner to express higher levels of proinflammatory cytokines (Fig. 1) (1,7). We are currently testing the hypothesis that metabolic endotoxemia, induced by a high-fat diet, together with OxCE, a component of atherosclerotic lesions, synergistically enhance vascular inflammation.
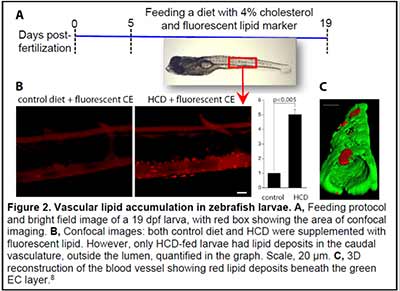
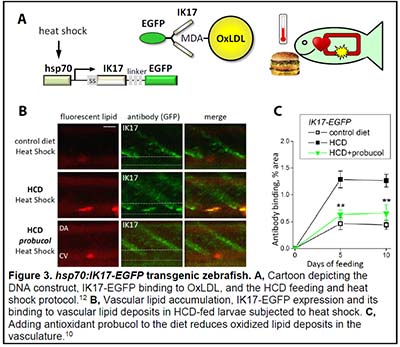
We have become fascinated by the zebrafish animal model because of the optical transparency of zebrafish larvae and the possibility to observe developmental, physiologic and pathologic processes in live animals. We have developed imaging and analytic techniques to study in vivo vascular lipid accumulation and leukocyte recruitment, endothelial disorganization and vascular permeability, and lipoprotein oxidation (Figs. 2 and 3) (8-13). In collaboration with Drs. Witztum and Tsimikas, we developed a transgenic zebrafish with conditional expression of the malondialdehyde (MDA)-specific human antibody IK17 conjugated with EGFP (Fig. 3) (10). The IK17-EGFP transgene was placed under control of the hsp70 promoter. A heat shock of such a hsp70:IK17-EGFP zebrafish induced transient secretion of IK17-EGFP and enabled us to image MDA epitopes at specific time points of vascular lesion development and test protective roles of antioxidants and regression diets (10).
We are also developing genetic models of lipid disorders. Our apoc2 knockout zebrafish display a strong hypertriglyceridemia phenotype and pancreas growth abnormalities (14). New transgenic lines overexpressing zebrafish pcsk9 and apoa1 and human ALOX15, as well as the line deficient in lpl are currently being propagated and analyzed.
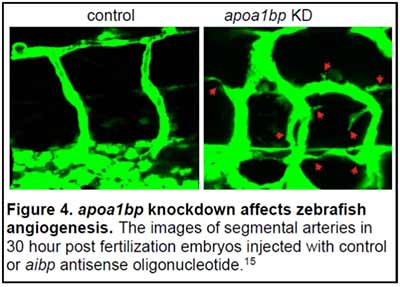 ApoA-I Binding Protein (AIBP, human gene APOA1BP) is a secreted, 29 kDa protein, which is ubiquitously expressed in many cell types and tissues. We discovered that extracellular AIBP accelerated cholesterol efflux from endothelial cells (EC) to HDL, the process mediated by the cellular cholesterol transporter ABCG1 (15). AIBP/HDL-mediated cholesterol efflux disrupted lipid rafts in the EC plasma membrane, which in turn interfered with VEGFR2 signaling and inhibited angiogenesis. We found that in embryonic zebrafish, the AIBP-mediated cholesterol efflux was essential for proper angiogenesis. The knockdown of AIBP in zebrafish resulted in dysregulated angiogenesis, characterized by excessive branching and sprouting of growing blood vessels (Fig. 4). In a gain-of-function experiment, ectopic expression of AIBP inhibited segmental artery sprouting from the dorsal aorta (15).
ApoA-I Binding Protein (AIBP, human gene APOA1BP) is a secreted, 29 kDa protein, which is ubiquitously expressed in many cell types and tissues. We discovered that extracellular AIBP accelerated cholesterol efflux from endothelial cells (EC) to HDL, the process mediated by the cellular cholesterol transporter ABCG1 (15). AIBP/HDL-mediated cholesterol efflux disrupted lipid rafts in the EC plasma membrane, which in turn interfered with VEGFR2 signaling and inhibited angiogenesis. We found that in embryonic zebrafish, the AIBP-mediated cholesterol efflux was essential for proper angiogenesis. The knockdown of AIBP in zebrafish resulted in dysregulated angiogenesis, characterized by excessive branching and sprouting of growing blood vessels (Fig. 4). In a gain-of-function experiment, ectopic expression of AIBP inhibited segmental artery sprouting from the dorsal aorta (15).
This new mechanistic link between cholesterol metabolism and angiogenesis opens numerous avenues to study the role of cholesterol efflux in general and of AIBP in particular in angiogenesis during development, in post-ischemic heart, when revascularization of the myocardium is beneficial to the patient, and in cancer, where inhibition of tumor vascularization is an important therapeutic strategy. We also plan to study the role of AIBP in lipid metabolism and in the development of atherosclerosis and other chronic inflammatory diseases. We are captivated by the prospect of AIBP as a therapy. Administration of recombinant AIBP may prevent hyper-inflammatory response in acute conditions as well as restrict chronic inflammatory responses. Elucidating mechanisms of anti-inflammatory responses to AIBP treatment and exploring its beneficial effects in various animal models of acute and chronic inflammatory diseases is a major focus of our studies.