Wei Ying Lab
- Our Team
- Research
- Publications
 The initial hypothesis of this project was that ferroptosis may occur in hepatocytes, resulting in the development of obesity-related liver steatosis and fibrosis. However, it is not true in my studies....but we found a more interesting story:
The initial hypothesis of this project was that ferroptosis may occur in hepatocytes, resulting in the development of obesity-related liver steatosis and fibrosis. However, it is not true in my studies....but we found a more interesting story:
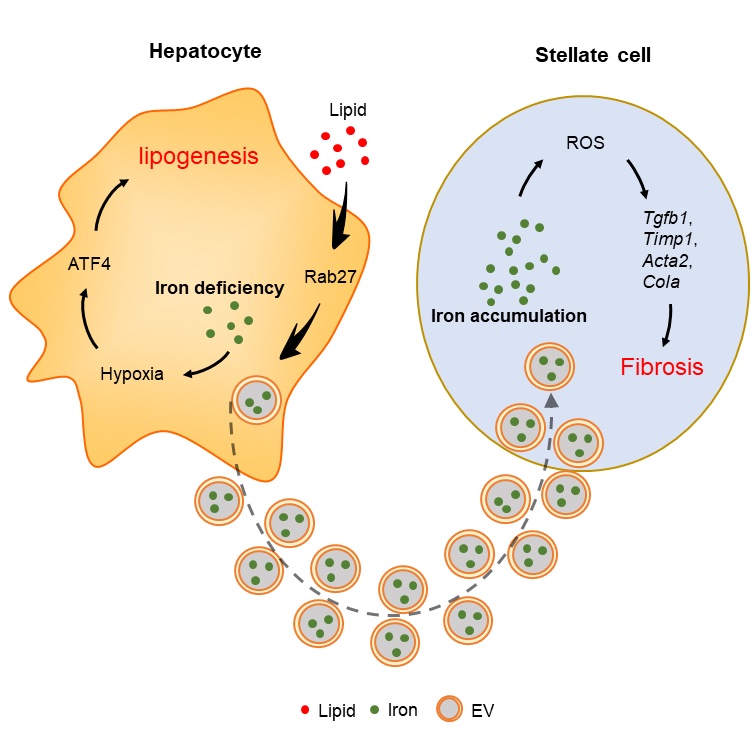
Non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) are characterized by iron-deficient hepatocytes and iron overload in hepatic stellate cells (HSCs). Iron deficiency enhances hepatocyte lipogenesis and insulin resistance through HIF2a-ATF4 signaling. Elevated secretion of iron-containing hepatocyte extracellular vesicles (EVs), which are normally cleared by Kupffer cells, accounts for hepatocyte iron deficiency and HSC iron overload in NAFLD/NASH livers. Iron accumulation results in overproduction of reactive oxygen species that promote HSC fibrogenic activation. Conversely, blocking hepatocyte EV secretion or depleting EV iron cargo restores liver iron homeostasis, concomitant with mitigation of NAFLD/NASH-associated liver steatosis and fibrosis.
 We love macrophages and love more on their "weapons"! Among these, microRNA-690 is a good one. We depicted how Kupffer cells use miR-690 to mediate the functions of hepatocytes and hepatic stellate cells in the healthy vs. NAFLD/NASH livers:
We love macrophages and love more on their "weapons"! Among these, microRNA-690 is a good one. We depicted how Kupffer cells use miR-690 to mediate the functions of hepatocytes and hepatic stellate cells in the healthy vs. NAFLD/NASH livers:
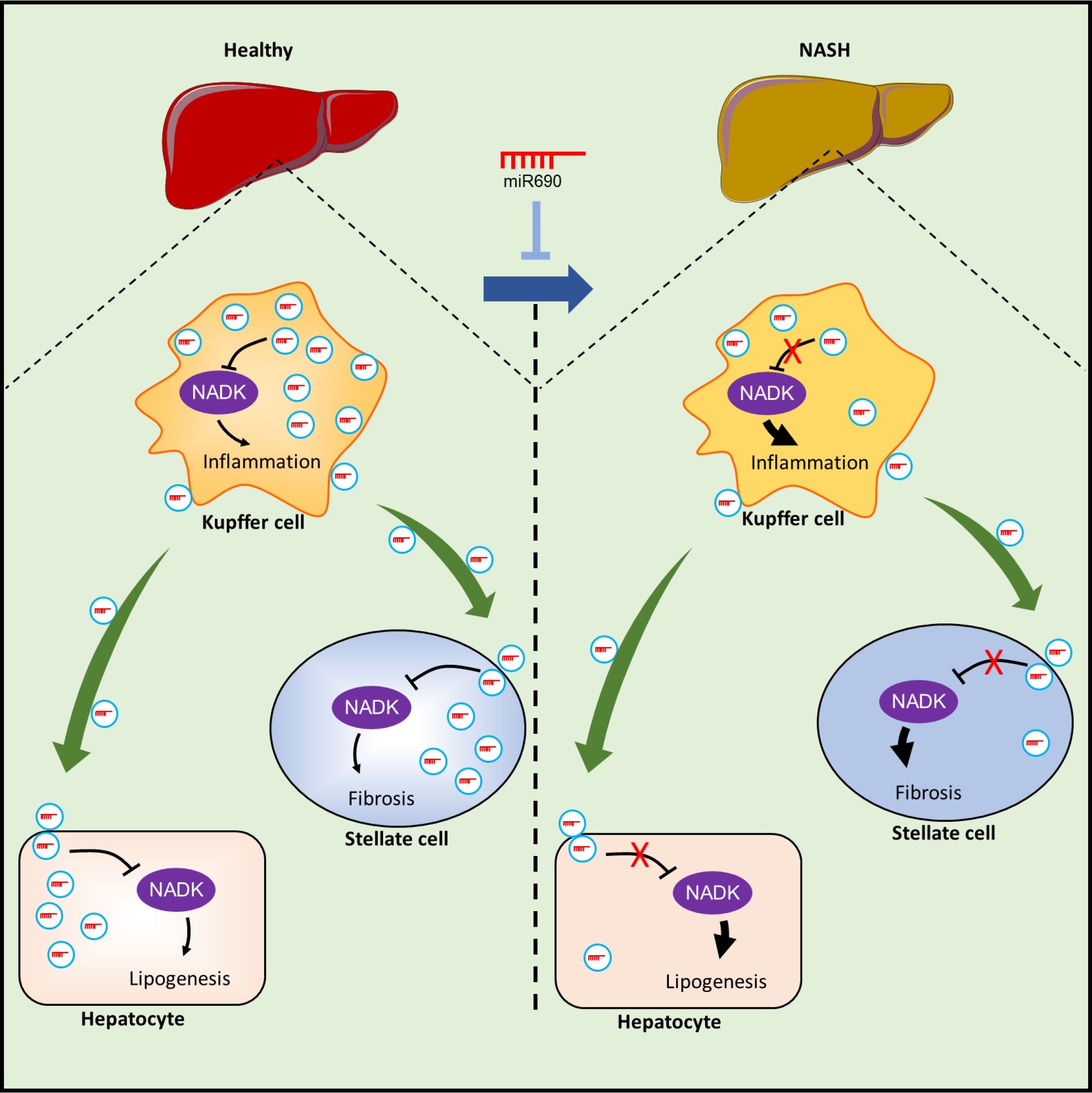
Kupffer cells (KCs) produce endogenous miR-690 and, via exosome secretion, shuttle this miRNA to other liver cells, such as hepatocytes, recruited hepatic macrophages (RHMs), and hepatic stellate cells (HSCs). miR-690 directly inhibits fibrogenesis in HSCs, inflammation in RHMs, and de novo lipogenesis in hepatocytes. When an miR-690 mimic is administered to NASH mice in vivo, all the features of the NASH phenotype are robustly inhibited. During the development of NASH, KCs become miR-690 deficient, and miR-690 levels are markedly lower in mouse and human NASH livers than in controls. KC-specific KO of miR-690 promotes NASH pathogenesis. A primary target of miR-690 is NADK mRNA, and NADK levels are inversely proportional to the cellular miR-690 content.
Again, we love macrophages!
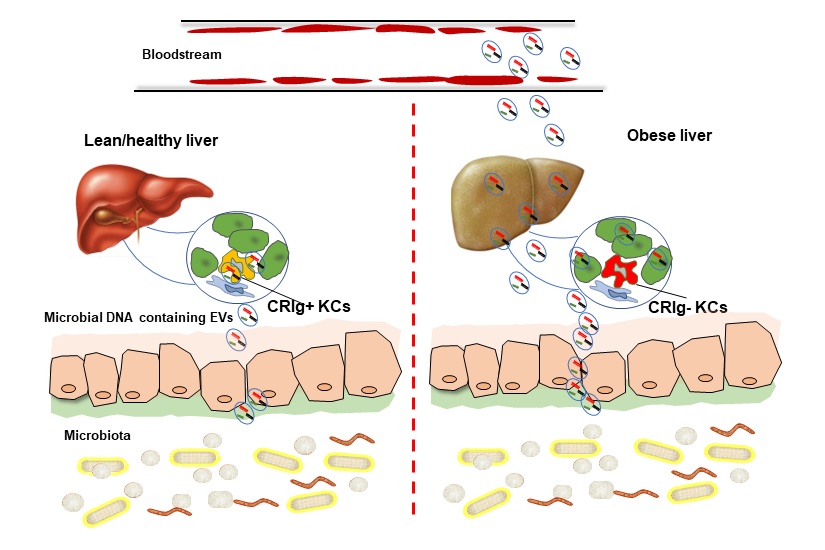 The bugs in your gut could be your good friends when you are lean and healthy. Their microbial DNA wrapped by extracellular vesicles (EVs) can be leaked into your blood and organs when overweight/obese. Kupffer cells are the key component of complement immunity, and CRIg is one of the critical "weapons" of KCs to phagocytize C3-opsonized bacterial products from the bloodstream. However, KCs lose CRIg in obese livers, thus allowing the spread of microbial DNA containing EVs from the gut lumen into distal host organs. Accumulation of microbial DNA in host cells results in inflammation and insulin resistance.
The bugs in your gut could be your good friends when you are lean and healthy. Their microbial DNA wrapped by extracellular vesicles (EVs) can be leaked into your blood and organs when overweight/obese. Kupffer cells are the key component of complement immunity, and CRIg is one of the critical "weapons" of KCs to phagocytize C3-opsonized bacterial products from the bloodstream. However, KCs lose CRIg in obese livers, thus allowing the spread of microbial DNA containing EVs from the gut lumen into distal host organs. Accumulation of microbial DNA in host cells results in inflammation and insulin resistance.
The next question: what causes the loss of CRIg in KCs in obesity?
 With the inspiration of insulin-sensitizing effects of lean tissue macrophage-derived exosomal miRNAs, we thought lean hepatocyte-secreted EVs might do similar work. Another reason is that it is so much easier to get lots of hepatocyte-derived EVs than macrophages! However, we didn't find this expectation from lean hepatocyte-EVs. I remember that in our weekly group meeting, Kamya, who was a postdoc from Dr. Olefsky's group, presented some interesting phenotypes showing a short period HFD feeding promoted hepatocyte insulin responses. These inspire us to another hypothesis for this project: hepatocytes may try to save systemic insulin sensitivity at the beginning of overloading nutrients by secretion of exosomal miRNAs? Here are what we found (Nat Metabol, 2021):
With the inspiration of insulin-sensitizing effects of lean tissue macrophage-derived exosomal miRNAs, we thought lean hepatocyte-secreted EVs might do similar work. Another reason is that it is so much easier to get lots of hepatocyte-derived EVs than macrophages! However, we didn't find this expectation from lean hepatocyte-EVs. I remember that in our weekly group meeting, Kamya, who was a postdoc from Dr. Olefsky's group, presented some interesting phenotypes showing a short period HFD feeding promoted hepatocyte insulin responses. These inspire us to another hypothesis for this project: hepatocytes may try to save systemic insulin sensitivity at the beginning of overloading nutrients by secretion of exosomal miRNAs? Here are what we found (Nat Metabol, 2021):
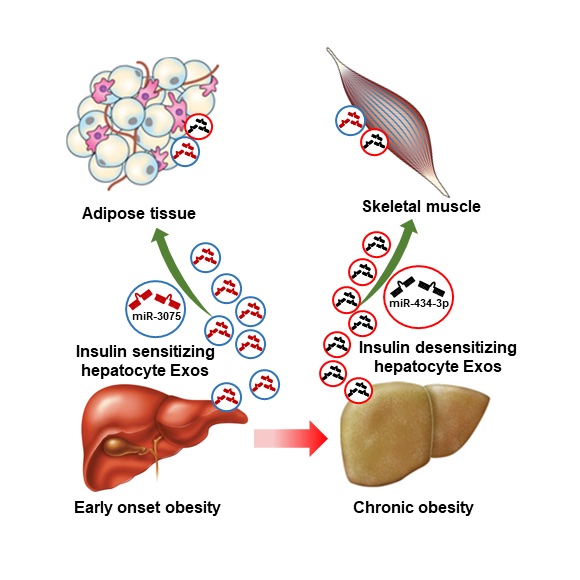
Our recent findings have demonstrated that in early onset obesity (4 weeks high-fat diet; 4wks HFD), hepatocytes exert profound compensatory improvement on peripheral insulin sensitivity through secreting extracellular miR-3075 which elevates insulin sensitivity in adipose tissue and skeletal muscle. In chronic obesity (16-18wks HFD), hepatocyte miR-3075 abundance was diminished, concomitant with greatly increased insulin resistance.